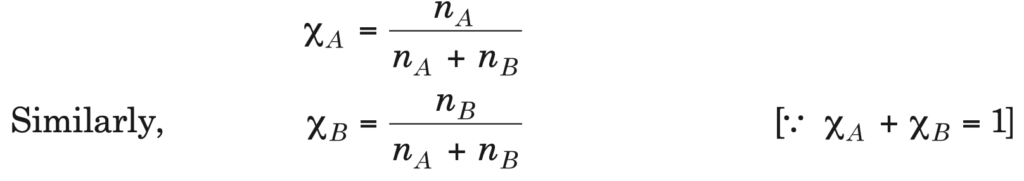We are Launching Live Zoom Classes for 9th and 10th-grade Students. The first batch is from 7th April 2025. Register for a Free demo class.
Chapter 1: Solutions (CBSE Class 12)
Introduction
- In our everyday life, we rarely come across pure substances. Most of them are mixtures of two or more substances.
- The use of a particular solution in everyday life depends on its composition. For example, brass is a homogeneous mixture of copper and zinc, German silver is a homogeneous mixture of copper, zinc, and nickel, while bronze is that of copper and tin.
What is a solution?
Solution is a homogeneous mixture of two or more substances in same or different physical phases. The substances forming the solution are called components of the solution. On the basis of number of components a solution of two components is called binary solution.
Solute and Solvent
In a binary solution, the solvent is the component that is present in large quantity, while the other component present in small quantity is known as the solute.
Types of Solutions
(A) Based on the physical state of the solute and the solvent
The following types of solutions are seen on the basis of the physical state of the solute and the solvent.
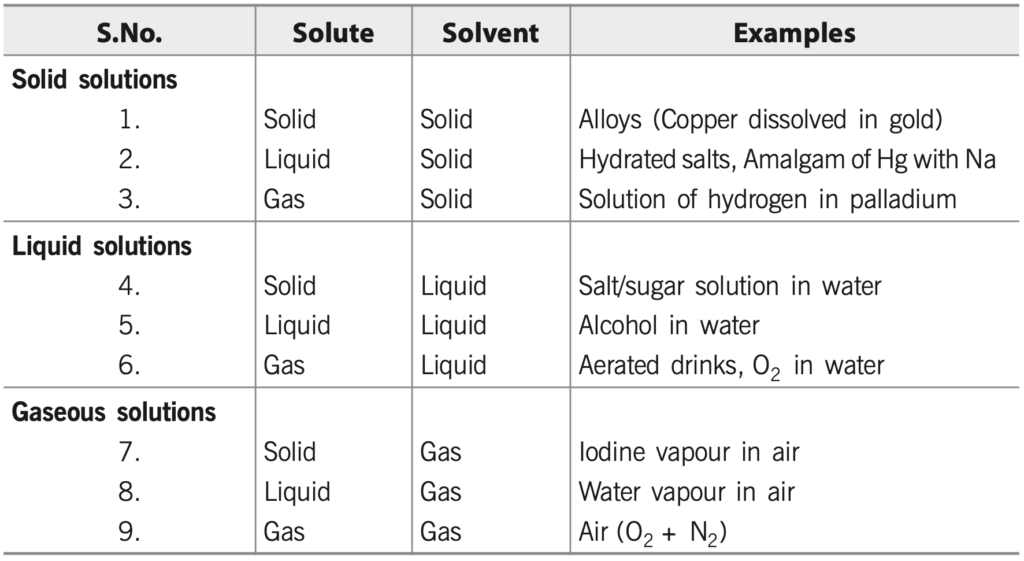
If water is used as a solvent, the solution is called an aqueous solution, and if not, the solution is called a non-aqueous solution.
(B) Based on the amount of solute dissolved in a solvent
Depending upon the amount of solute dissolved in a solvent, we have the following types of solutions:
- Unsaturated solution: A solution in which more solute can be dissolved without raising the temperature is called an unsaturated solution.
- Saturated solution: A solution in which no more solute can be dissolved further at a given temperature is called a saturated solution.
- Supersaturated solution: A solution that contains more solute than that would be necessary to saturate it at a given temperature is called a supersaturated solution.
Expressing Concentration of Solutions
Concentration of Solutions
The concentration of a solution is defined as the relative amount of solute present in a solution. On the basis of the concentration of the solution, there are two types of solutions:
- Dilute solution: Solution containing relatively very small quantity of solute.
- Concentrated solution: Solution containing a relatively very large quantity of solute.
Methods of Expressing Concentration of Solutions
Various expressions for the concentrations of solutions can be summarised as:
1. Percentage by weight (w/w %): It is defined as the amount of solute present in 100 g of solution
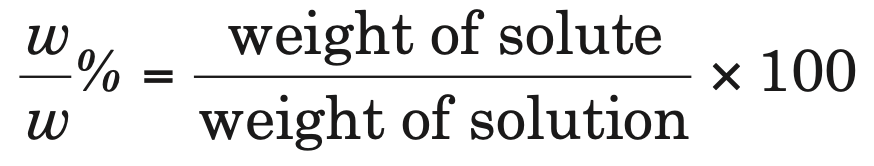
2. Percentage by volume (v/V %): It is defined as the volume of solute present in 100 mL of solution,
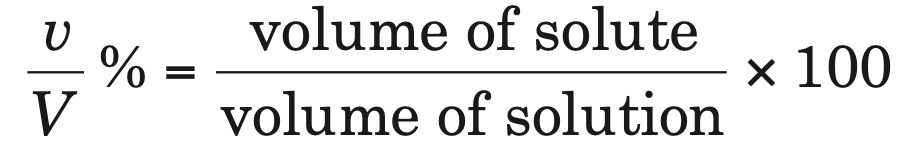
3. Percentage of mass by volume (w/V %): It is defined as the weight of solute present in 100 mL of solution.
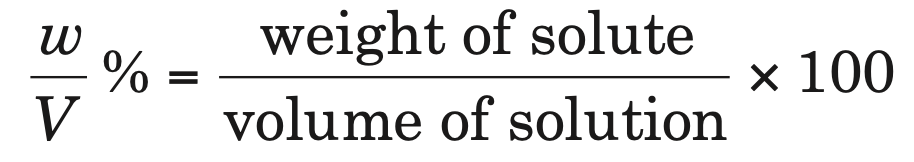
4. Mole fraction (χ): It is defined as the ratio of the number of moles of a component to the total number of moles of all the components in solution. For a binary solution, if the number of moles of A and B are nA and nB respectively, the mole fraction of A will be: