We are Launching Live Zoom Classes for 9th and 10th-grade Students. The first batch is from 7th April 2025. Register for a Free demo class.
Carbon and Its Compounds
Chapter 4: Carbon and Its Compounds
Introduction:
Two or more elements combine to form a compound.
There are two types of compounds: Organic Compounds and Inorganic Compounds.
Organic compounds are made up of carbon and hydrogen (generally known as hydrocarbons)
Covalent Bond
- The bond formed by sharing a pair of electrons between two atoms is known as Covalent Bond.
- Carbon forms covalent bonds.
- Carbon exists in two forms- as a free state and as a combined state.
- The free form of carbon is found in graphite, diamond and fullerene. In the combined state, carbon exists as Carbon-dioxide, Glucose, Sugar etc.
Allotropes of Carbon
- Different forms of an element that have the same chemical properties but different physical properties are known as Allotropes.
- There are three allotropes of carbon- diamond, graphite and fullerene.
Diamond
- Diamond exists as a three-dimensional network with strong carbon-carbon covalent bonds.
- Diamonds are hard in nature with high melting points.
- It shines in the presence of light (due to its high refractive index and total internal reflection).
- It is a bad conductor of electricity.
- The most common use of diamonds is in making jewellery.
- It is also used in cutting and drilling tools.
Graphite
- Graphite is made from weak Van der Waal forces.
- Each carbon atom is bonded with the other three carbon atoms to form hexagonal rings.
- It serves as a good conductor of heat and electricity.
- It is used as a dry lubricant for machine parts (generally at higher temperatures where liquid lubricants cannot be used).
- It is also used in lead pencils.
Fullerene
- It is a hollow cage that exists in the form of a sphere made up of several hexagonal rings.
- Its structure is similar to fullerene.
- Along with hexagonal rings, sometimes pentagonal or heptagonal rings are also present.
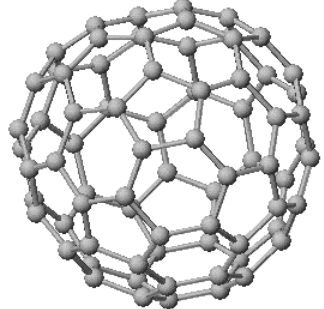
Fig.1 Structure of fullerene
Two Important Properties of Carbon
- Catenation and tetravalency are the two important properties of carbon.
- Catenation is a property of carbon by which carbon atoms can link one another via the covalent bonds and can form long chains, closed rings or branched chains etc.
- Carbon atoms can be linked by single, double or triple bonds.
- Carbon has a valency of 4 due to which it is known to have tetravalency.
- Due to this one carbon atom can bond with the other 4 carbon atoms, with other atoms also such as Oxygen, Nitrogen etc.
Hydrocarbons
- Compounds that are made up of carbon and hydrogen are known as Hydrocarbons.
- There are two types of hydrocarbons found – Saturated Hydrocarbons and Unsaturated Hydrocarbons.
- Saturated Hydrocarbons consist of single bonds between the carbon atoms.
- For Example, Alkanes. Alkanes are saturated hydrocarbons represented by a formula, CnH2n+2.
- Unsaturated Hydrocarbons are the ones with double or triple bonds between the carbon atoms.
- For Example, Alkenes and Alkynes. Alkenes are represented as CnH2n whereas alkynes are represented as CnH2n-2.
- Some saturated hydrocarbons and unsaturated hydrocarbons are represented as –

Fig.2. Saturated hydrocarbons
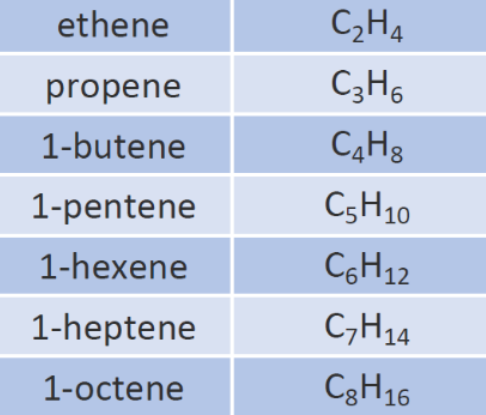
Fig. 3. Unsaturated hydrocarbons
- The structure of hydrocarbons can be represented in the form of electron dot structure as well as open structures as shown below-
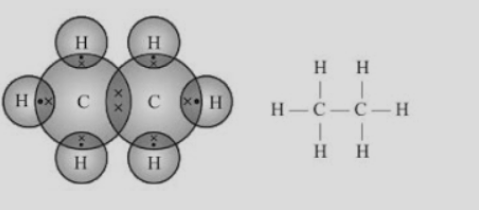
Fig.4. Electron dot structure and open structure of ethane
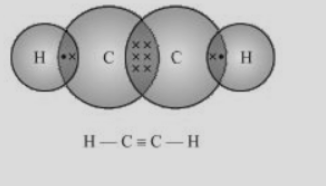
Fig.5. Electron dot structure and open structure of ethyne
Carbon Compounds based on the structure
- Carbon Compounds can be classified as straight-chain compounds, branched-chain compounds and cyclic compounds. They are represented as –
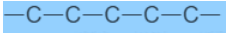
Fig.6. Straight chain carbon compound
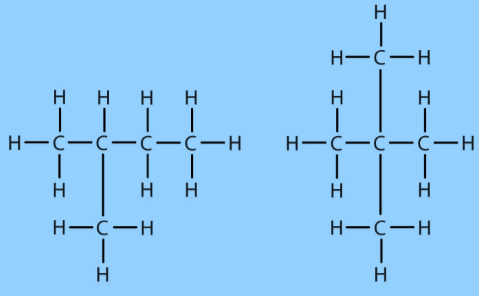
Fig.7. Branched-chain compounds
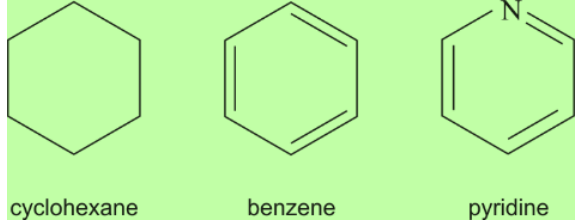
Fig.8. Cyclic carbon compounds
Functional Groups
- One of the hydrogen atoms in hydrocarbons can be replaced by other atoms also called hetero-atoms (like halogens, oxygen, nitrogen, and sulphur) such that the valency of carbon remains satisfied.
- Hetero-atoms or their groups, when present, imparts special property to the hydrocarbon.
- The atoms or groups of atoms, which decide the properties of the hydrocarbon compound are known as Functional Groups.
- For Example, Cl, Br, -OH (alcohol), Aldehyde, Ketone, Carboxylic Acid, etc.
Homologous Series
- It is a series of compounds, having similar chemical properties, due to the presence of the same functional group in a chain of carbon.
- For example, CH3OH, C2H5OH, C3H7OH and C4H9OH are all have the same functional group (-OH) and have very similar chemical properties.
- Such a series of compounds is called homologous series.
- Chemical properties of compounds in a homologous series remains similar.
- Successive members of homologous series differ by a -CH2 unit.
- Mass of one -CH2 unit is 14 u.
- Therefore each successive member of a homologous series has 14 u more molecular mass than its previous member.
- Gradation in physical properties:
- It means a regular and gradual change in the physical properties of compounds in a homologous series.
- As molecular mass increases in any homolous series, melting point and boiling point of compounds increases.
- Also, other physical properties like solubility on compound in a particular solvnet also show similar gradation.
- However, chemical properties remains similar in a homologous series.
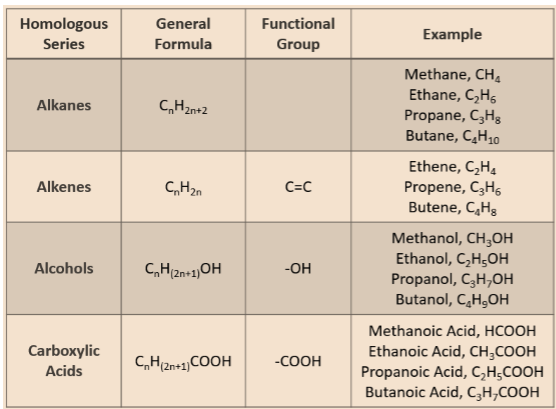
Fig.9. Homologous series
Nomenclature of Carbon Compounds
- First of all, identify the number of carbon atoms in compounds. And in it identify the longest chain.
- Then the functional group can be indicated by suffix or prefix.
- Cyclic hydrocarbon is designated by the prefix cyclo.
- If there are two or more different substituents they are listed in alphabetical order.
- If the same substituent occurs more than once, the location of each point on which the substituent occurs is given
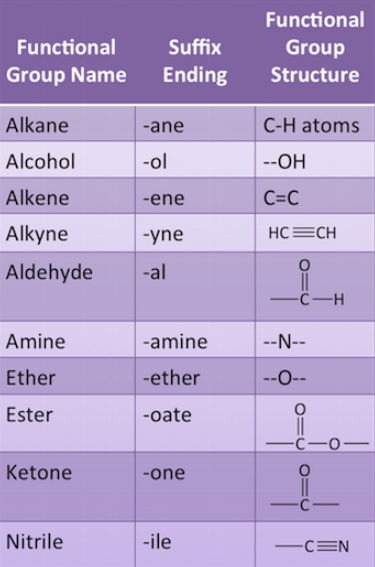
Fig.10. Different functional groups
Chemical Properties of Carbon Compounds
- Combustion
- Carbon (coal, coke, etc) along with its compound is used as a fuel as it burns in presence of oxygen to release energy and carbon dioxide
- Even diamond and graphite burns in oxygen to give carbon dioxide and energy.
- Saturated hydrocarbons produce blue and non-sooty flame whereas unsaturated hydrocarbons produce a yellow sooty flame.
- Fuels like coal and petroleum have some amount of nitrogen and sulphur impurity in them and their combustion results in formation of oxides of nitrogen and oxides of sulphur.
- Nitrogen oxides and sulphur oxides are major pollutants in the environment.
CH4+2O2-> CO2+2H2O
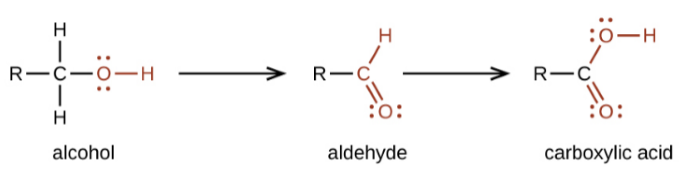
- Oxidation
- Carbon compounds oxidise easily on combustion to form oxides and water.
- With controlled oxidation we can get several industrially important products by using oxidising agents.
- For example, Alcohol can be oxidised to aldehydes whereas aldehydes, in turn, can be oxidised to carboxylic acid.
- Oxidising agents such as potassium permanganate and potassium dichromate can be used for oxidation.
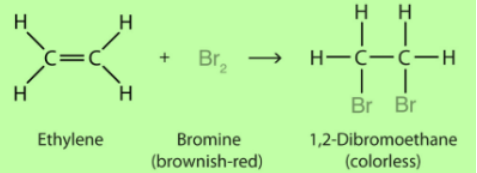
- Addition Reaction
- It is the property of unsaturated hydrocarbon.
- Hydrogenation of vegetable oil is an example of an additional reaction.
- Addition of hydrogen to unsaturated hydrocarbons in presence of catalysts such as nickel or palladium is an example of an addition reaction.
- This converts the oil into vanaspati ghee.
- Oils or fats having unsaturated carbon chains are healthy and should be chosen for cooking.
- Vegetable oils are unsaturated while animal fat and vanaspati ghee are saturated.
- Addition of bromine to ethylene is another example of an addition reaction.
- Substitution Reaction
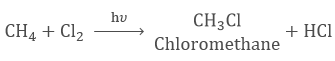
- When one atom in hydrocarbon is replaced by other atoms like chlorine, bromine, etc. this is known as a Substitution Reaction.
- Saturated hydrocarbons are the least reactive and they undergo substitution reactions.
- Chlorine atom substitutes hydrogen atom one by one in saturated hydrocarbon in presence of sunlight.
- It is called substitution reaction because one type of atom or a group of atoms takes the place of another.
Important Carbon Compounds: Ethanol and Ethanoic Acid
Ethanol and ethanoic acids are two commercially important carbon compounds.
- Properties of Ethanol
- Ethanol is a volatile liquid at room temperature.
- It has low melting and boiling point.
- It is active ingredient of all alcoholic drinks like wine, whiskey, rum, etc.
- It is also a very good solvent and used in medicines such as tincture iodine, cough syrups and tonics.
- It is soluble in water.
- Consumption of dilute ethanol (wine, rum, whiskey, etc.) is a socially widespread practice, though it causes drunkenness, and may leads to many health problems.
- Intake of even small quantity of pure ethanol (absolute alcohol, i.e. no water added) can be leathal.
- It reacts with sodium to form sodium ethoxide.
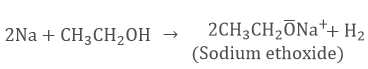
This reaction is used to test the presence of ethanol by the evolution of hydrogen gas.
- Dehydration of ethanol in presence of hot and concentrated sulphuric acid forms ethene.
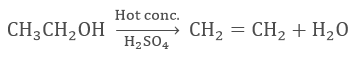
- Properties of Ethanoic Acid
- Commonly called as acetic acid.
- Ethanoic acid is a colourless liquid.
- It belongs to a group of acids called carboxylic acids.
- 5% to 8% solution of acetic acid in water is called vineger.
- Pure ethanoic acid freezes like ice at around 16.6 degree celcius during cold winter like ice, hence it is also known by the name Glacial Acetic Acid.
- Carboxylic acids including acetic acid are weak acids.
- Ethanoic Acid/Acetic acid when reacts with ethanol forms an ester. Ester can be identified by its sweet and fruity smell.

- Ester is used in making perfumes and flavouring essence like vanilla essence, etc.
- The reaction of esters with a strong base is used to form soap. This is known as Saponification Reaction.
- Acetic acid also reacts with a strong base like sodium hydroxide to form sodium acetate and water.
NaOH + CH3COOH + CH3COONa + H2O
- Ethanoic acid reacts with carbonates and hydrogencarbonates to give rise to a salt (acetate), carbon dioxide and water.
Soaps and Detergents
- Sodium or potassium salt of a long-chain carboxylic acid is known as Soap.
- They work most effectively in soft water.
- Detergents are sulphonate or ammonium salt of a long-chain of carboxylic acid.
- They can work effectively on soft as well as hard water.
Cleansing Action of Soaps and Detergents
- When soap is dissolved in water, it ionises, one end of soap molecule is anion and other end consists of long hydrocarbon chain.
- The ionic end of soap is hydrophilic , while the long hydrocarbon chain is hydrophobic in nature.
- The hydrophobic part of soaps and detergents are soluble in grease and the hydrophilic part is soluble in water.
- Thus, the cleansing action of soaps and detergents is due to the ability to emulsify oil or grease and hold them in a suspension of water.
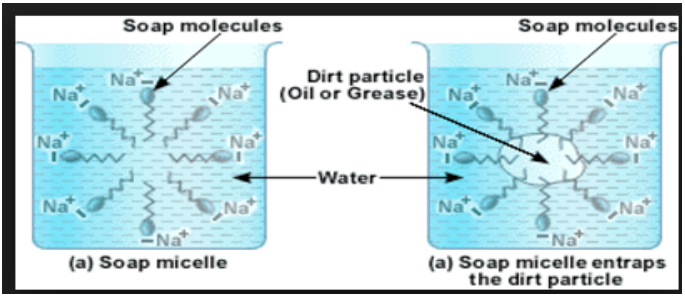
Micelle Formation
- When dirt and grease are mixed with soap water, soap molecules arrange them in tiny clusters known as Micelle.
- The hydrophilic part sticks to the water and forms the outer surface of the micelle and the hydrophobic part binds to oil and grease.
- This cluster formation is called miscelle.
- Oily and dirt is collected in the centre of the miscelle, remained suspended in water and is also rinsed away easily.