We are Launching Live Zoom Classes for 9th and 10th-grade Students. The first batch is from 7th April 2025. Register for a Free demo class.
Structure of The Atom
- It was known by 1900 that the atom was not a simple, indivisible particle but contained at least one sub-atomic particle – the electron identified by J.J. Thomson.
- Even before the electron was identified, E. Goldstein discovered the presence of protons which were positively charged.
- Protons had a charge, equal in magnitude but opposite in sign to that of the electron.
- Its mass was approximately 2000 times that of the electron.
- The mass of an electron is considered to be negligible and its charge is minus one.
- It seemed likely that an atom was composed of protons and electrons, mutually balancing their charges.
- It also appeared that the protons were in the interior of the atom, whereas electrons could easily be peeled off but not protons.
- Dalton’s atomic theory suggested that the atom was indivisible and indestructible.
- But the discovery of two fundamental particles (electrons and protons) inside the atom, led to the failure of this aspect of Dalton’s Atomic Theory.
- It was then considered necessary to know how electrons and protons are arranged within an atom.
- To explain this, many scientists proposed various atomic models.
- J.J. Thomson was the first one to propose a model for the structure of an atom
Thomson’s Model of an Atom
- J.J. Thomson (1856-1940), was awarded the Nobel Prize in physics for his work on the discovery of electrons.
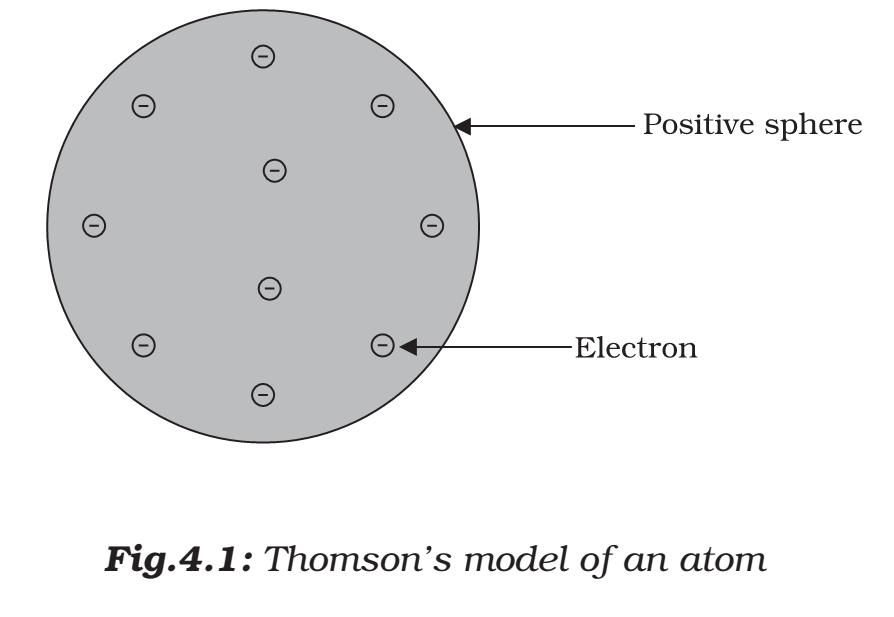
- Thomson proposed the model of an atom to be similar to that of a watermelon.
- The positive charge in the atom is spread all over like the red edible part of the watermelon, while the electrons are studded in the positively charged sphere, like the seeds in the watermelon
Thomson proposed that:
- An atom consists of a positively charged sphere and the electrons are embedded in it.
- The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
- Although Thomson’s model explained that atoms are electrically neutral, the results of experiments carried out by other scientists could not be explained by this model, as we will see below.
Rutherford’s Model of an Atom
- E. Rutherford was known as the ‘father’ of nuclear physics.
- He is famous for his work on radioactivity and the discovery of the nucleus of an atom with the gold foil experiment. He got the Nobel Prize in chemistry in 1908.
- Ernest Rutherford was interested in knowing how the electrons are arranged within an atom.
- Rutherford designed an experiment for this.
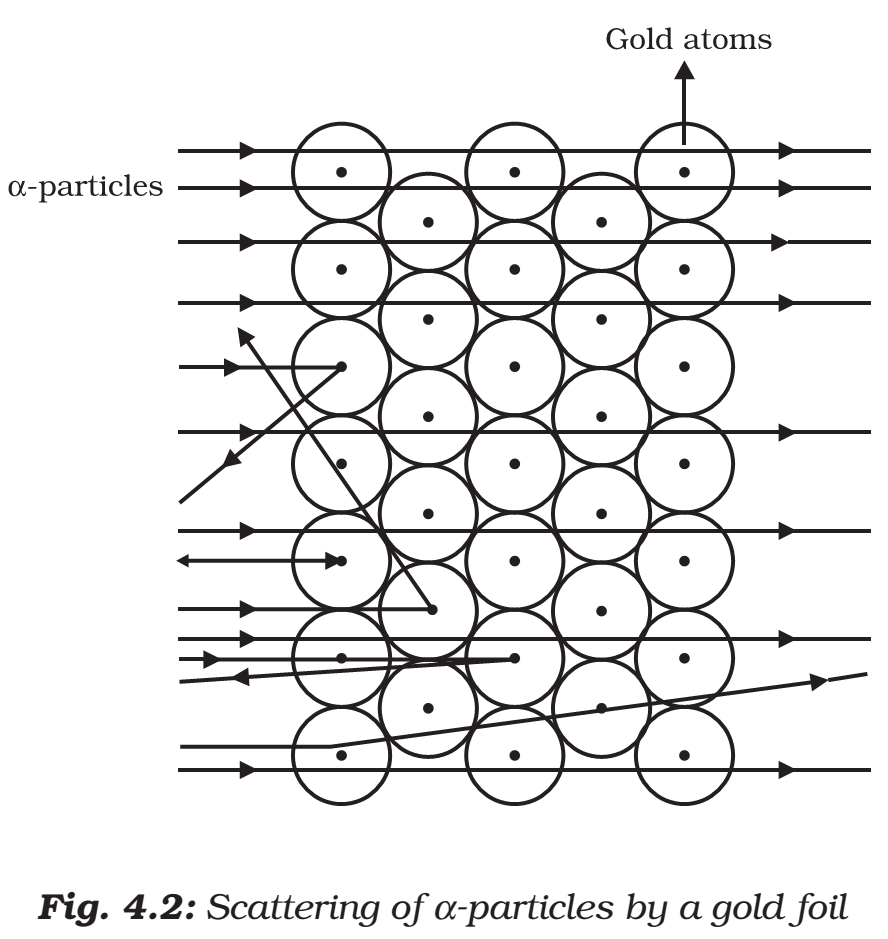
- In this experiment, fast-moving alpha (α)-particles were made to fall on a thin gold foil.
- (α)-particles are doubly-charged helium ions.
- Since they have a mass of 4 u, the fast-moving α-particles have a considerable amount of energy.
- It was expected that α-particles would be deflected by the sub-atomic particles in the gold atoms.
- Since the α-particles were much heavier than the protons, he did not expect to see large deflections.
- But, the α-particle scattering experiment gave unexpected results.
- The following observations were made:
- Most of the fast-moving α-particles passed straight through the gold foil.
- Some of the α-particles were deflected by the foil by small angles.
- Surprisingly one out of every 12000 particles appeared to rebound.
- In the words of Rutherford, “This result was almost as incredible as if you fire a 15-inch shell at a piece of tissue paper and it comes back and hits you”.
Conclusions drawn from the α-particle scattering experiment
- Most of the space inside the atom is empty because most of the α-particles pass through the gold foil without getting deflected.
- Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
- A very small fraction of α-particles were deflected indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.
- From the data, he also calculated that the radius of the nucleus is about 105 times less than the radius of the atom.
- Based on his experiment, Rutherford put forward the nuclear model of an atom, which had the following features:
- There is a positively charged centre in an atom called the nucleus.
- Nearly all the mass of an atom resides in the nucleus.
- The electrons revolve around the nucleus in well-defined orbits.
- The size of the nucleus is very small compared to the size of the atom.

Drawbacks of Rutherford’s model of the atom
- The orbital revolution of the electron is not expected to be stable.
- Any particle in a circular orbit would undergo acceleration.
- During acceleration, charged particles would radiate energy.
- Thus, the revolving electron would lose energy and finally fall into the nucleus.
- If this were so, the atom would be highly unstable and hence matter would not exist in the form that we know. We know that atoms are quite stable.
Neutrons
- In 1932, J. Chadwick discovered another subatomic particle which had no charge and a mass nearly equal to that of a proton. It was eventually named as neutron.
- Neutrons are present in the nucleus of all atoms, except hydrogen.
- In general, a neutron is represented as ‘n’.
- The mass of an atom is therefore given by the sum of the masses of protons and neutrons present in the nucleus (The mass of electrons is quite negligible).
- Electron 🡺 J.J. Thomson
- Proton 🡺 E. Goldstein
- Neutron 🡺 J. Chadwick
- Nucleus 🡺 E. Rutherford (father of nuclear physics)
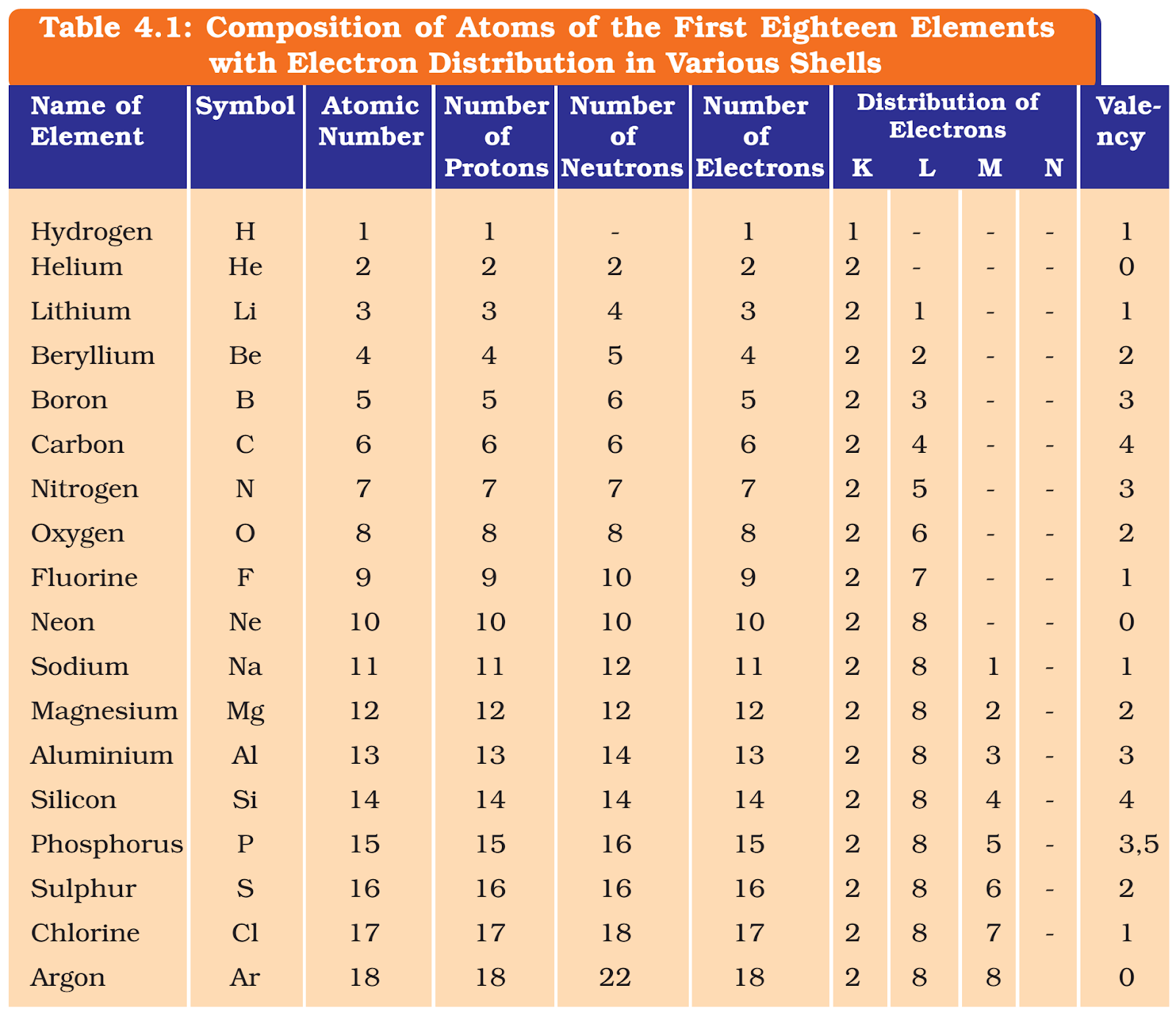
Atomic Number
- We know that protons are present in the nucleus of an atom.
- It is the number of protons in an atom, which determines its atomic number. It is denoted by ‘z’.
- All atoms of an element have the same atomic number, z.
- In fact, elements are defined by the number of protons they possess.
- For hydrogen, z = 1, because in a hydrogen atom, only one proton is present in the nucleus.
- Similarly, for carbon, z = 6.
- Therefore, the atomic number is defined as the total number of protons present in the nucleus of an atom.
Mass Number
- After studying the properties of the subatomic particles of an atom, we can conclude that the mass of an atom is practically due to protons and neutrons alone.
- These are present in the nucleus of an atom. Hence protons and neutrons are also called nucleons.
- Therefore, the mass of an atom resides in its nucleus.
- For example, the mass of carbon is 12 u because it has 6 protons and 6 neutrons, 6 u + 6 u = 12 u.
- Similarly, the mass of aluminium is 27 u (13 protons +14 neutrons).
- The mass number is defined as the sum of the total number of protons and neutrons present in the nucleus of an atom.
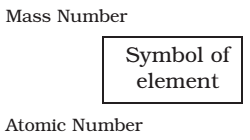
- The atomic number, mass number and symbol of the element are to be written as:
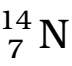
Isotopes
- In nature, a number of atoms of some elements have been identified, which have the same atomic number but different mass numbers.
- For example, take the case of hydrogen atom, which has three atomic species, namely protium (1H1), deuterium (2H1) and tritium (3H1).
- The atomic number of each one is 1, but the mass number is 1, 2 and 3, respectively.
- Other such examples are (i) carbon, 12C6 and 14C6 (ii) chlorine, 35Cl17 and 37Cl17, etc.
- On the basis of these examples, isotopes are defined as the atoms of the same element, having the same atomic number but different mass numbers.
- Therefore, we can say that there are three isotopes of hydrogen atoms, namely protium, deuterium and tritium.
- Each isotope of an element is a pure substance.
- Many elements consist of a mixture of isotopes.
- The chemical properties of isotopes are similar, but their physical properties are different.
- Chlorine occurs in nature in two isotopic forms, with masses 35 u and 37 u in the ratio of 3:1. Obviously, the question arises: what should we take as the mass of a chlorine atom?
- The mass of an atom of any natural element is taken as the average mass of all the naturally occurring atoms of that element.
- If an element has no isotopes, then the mass of its atom would be the same as the sum of protons and neutrons in it.
- But if an element occurs in isotopic forms, then we have to know the percentage of each isotopic form and then the average mass is calculated.
Applications of Isotopes
- Since the chemical properties of all the isotopes of an element are the same, normally we are not concerned about taking a mixture.
- But some isotopes have special properties which find them useful in various fields. Some of them are:
- An isotope of uranium is used as a fuel in nuclear reactors.
- An isotope of cobalt is used in the treatment of cancer.
- An isotope of iodine is used in the treatment of goitre.
Isobars
- Let us consider two elements — calcium, atomic number 20, and argon, atomic number 18.
- The number of electrons in these atoms is different, but the mass number of both these elements is 40.
- That is, the total number of nucleons is the same in the atoms of this pair of elements.
- Atoms of different elements with different atomic numbers, which have the same mass number, are known as isobars.
Bohr’s Model of Atom
- To overcome the objections raised against Rutherford’s model of the atom, Neils Bohr put forward the following postulates about the model of an atom:
- Only certain special orbits known as discrete orbits of electrons, are allowed inside the atom
- While revolving in discrete orbits the electrons do not radiate energy
- These orbits or shells are called energy levels. Energy levels in an atom are shown in the figure below.
- These orbits or shells are represented by the letters k,l,m,n,… or the numbers, n=1,2,3,4,….

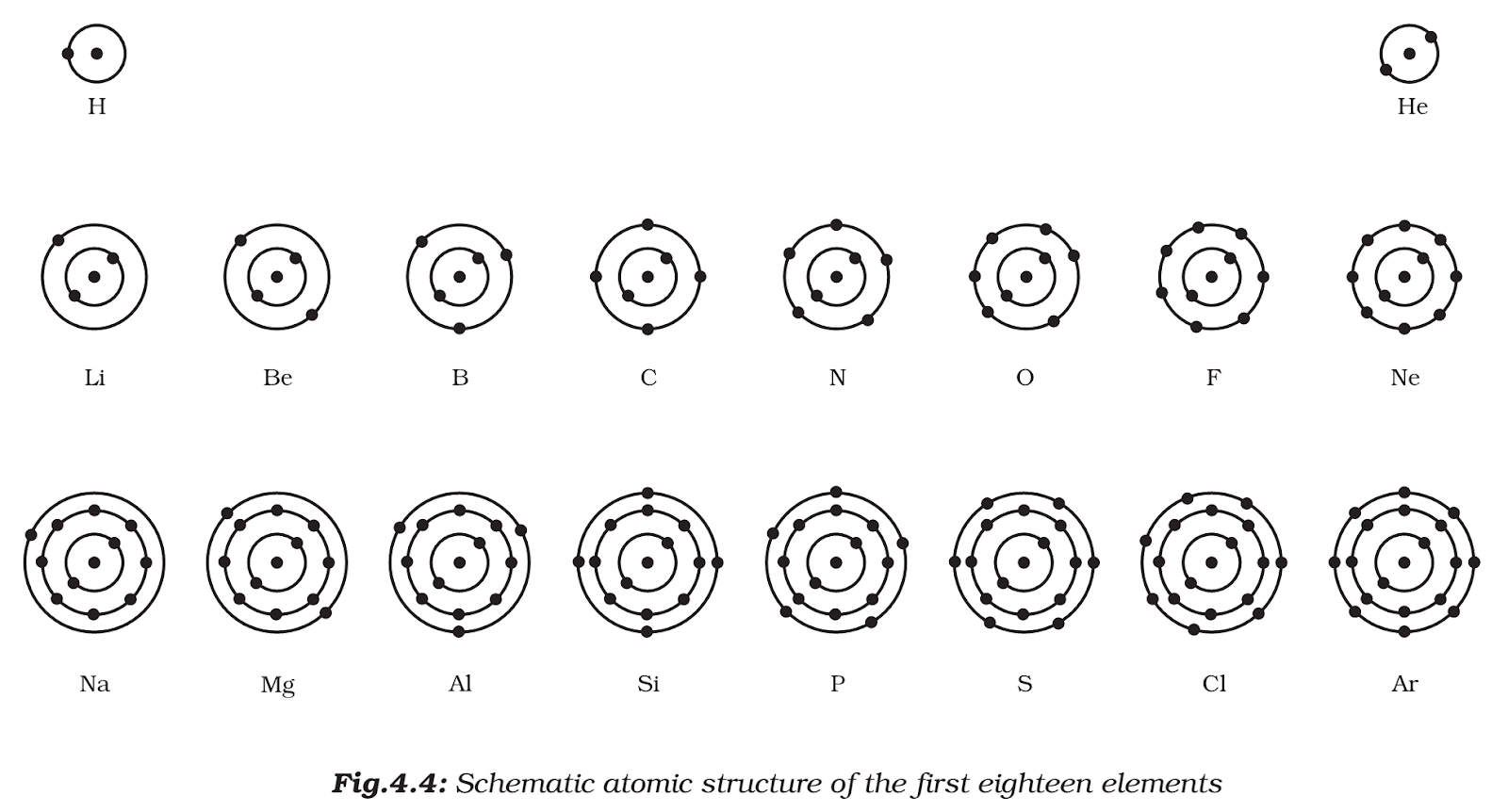
How are electrons distributed in different orbits (shells)?
- The distribution of electrons into different orbits of an atom was suggested by Bohr and Bury.
- The following rules are followed for writing the number of electrons in different energy levels or shells:
- the maximum number of electrons present in a shell is given by the formula 2n2, where ‘n’ is the orbit number or energy level index, 1, 2, 3, ….
- Hence the maximum number of electrons in different shells are as follows: first orbit or k-shell will be = 2 ×12 = 2, second orbit or l-shell will be = 2 ×22 = 8, third orbit or m-shell will be = 2 × 32 = 18, fourth orbit or n-shell will be = 2 ×42 = 32, and so on.
- The maximum number of electrons that can be accommodated in the outermost orbit is 8.
- Electrons are not accommodated in a given shell unless the inner shells are filled. That is, the shells are filled in a step-wise manner.
Valency
- The electrons present in the outermost shell of an atom are known as the valence electrons.
- The outermost shell of an atom can accommodate a maximum of 8 electrons.
- It was observed that the atoms of elements, having a completely filled outermost shell show little chemical activity. In other words, their combining capacity or valency is zero.
- Of these inert elements, the helium atom has two electrons in its outermost shell and all other elements have atoms with eight electrons in the outermost shell.
- The combining capacity of the atoms of other elements, that is, their tendency to react and form molecules, was thus explained as an attempt to attain a fully-filled outermost shell.
- An outermost shell, which had eight electrons was said to possess an octet.
- Atoms would thus react, so as to achieve an octet in the outermost shell.
- This was done by sharing, gaining or losing electrons.
- The number of electrons gained, lost or shared so as to make the octet of electrons in the outermost shell, gives us directly the combining capacity of the element, that is, the valency.
- For example, hydrogen/lithium/sodium atoms contain one electron each in their outermost shell, therefore each one of them can lose one electron. So, they are said to have a valency of one.
- The valency of magnesium and aluminium is two and three, respectively, because magnesium has two electrons in its outermost shell and aluminium has three electrons in its outermost shell.
- If the number of electrons in the outermost shell of an atom is close to its full capacity, then valency is determined differently.
- For example, the fluorine atom has 7 electrons in the outermost shell, and its valency could be 7.
- However, it is easier for fluorine to gain one electron instead of losing seven electrons.
- Hence, its valency is determined by subtracting seven electrons from the octet and this gives you a valency of one for fluorine.
- Therefore, an atom of each element has a definite combining capacity, called its valency.
Summary
- The chemical formula of a molecular compound is determined by the valency of each element.
- In ionic compounds, the charge on each ion is used to determine the chemical formula of the compound.
- Scientists use the relative atomic mass scale to compare the masses of different atoms of elements.
- Atoms of carbon-12 isotopes are assigned a relative atomic mass of 12 and the relative masses of all other atoms are obtained by comparison with the mass of a carbon-12 atom.
- The Avogadro constant 6.022 × 1023 is defined as the number of atoms in exactly 12 g of carbon-12.
- The mole is the amount of substance that contains the same number of particles (atoms/ ions/ molecules/ formula units etc.) as there are atoms in exactly 12 g of carbon-12.
- The mass of 1 mole of a substance is called its molar mass.
- Credit for the discovery of electron and proton goes to J.J. Thomson and E. Goldstein, respectively.
- J.J. Thomson proposed that electrons are embedded in a positive sphere.
- Rutherford’s alpha-particle scattering experiment led to the discovery of the atomic nucleus.
- Rutherford’s model of the atom proposed that a very tiny nucleus is present inside the atom and electrons revolve around this nucleus. The stability of the atom could not be explained by this model.
- Neils Bohr’s model of the atom was more successful. He proposed that electrons are distributed in different shells with discrete energy around the nucleus.
- If the atomic shells are complete, then the atom will be stable and less reactive.
- J. Chadwick discovered the presence of neutrons in the nucleus of an atom.
- So, the three sub-atomic particles of an atom are (i) electrons, (ii) protons and (iii) neutrons.
- Electrons are negatively charged; protons are positively charged, and neutrons have no charges.
- The mass of an electron is about 1/2000 times the mass of a hydrogen atom.
- The mass of a proton and a neutron is taken as one unit each.
- Shells of an atom are designated as k,l,m,n,….
- Valency is the combining capacity of an atom.
- The atomic number of an element is the same as the number of protons in the nucleus of its atom.
- The mass number of an atom is equal to the number of nucleons in its nucleus
- Isotopes are atoms of the same element, which have different mass numbers.
- Isobars are atoms having the same mass number but different atomic numbers.
- Elements are defined by the number of protons they possess.
Note: The above details have been compiled from the NCERT Science book of Class 9.



